IBISWorld Platform
Answer any industry question in minutes with our entire database at your fingertips.

Clinical trial support service providers have experienced significant growth in recent years, driven by a surge in demand for innovative therapies and rapid advancements in clinical and digital technologies. The growing complexity and volume of trials for treatments in areas such as oncology, rare diseases and advanced therapies, including cell and gene therapy, have heightened the need for efficient and specialized partners. As sponsors rush to bring new drugs and biologics to market, partnering with clinical trial service providers has become essential for managing the expanding demands of global patient recruitment, data management and regulatory compliance. Despite rising demand, operating pressures have created volatility, leading revenue to weaken at a CAGR of 1.9% to an estimated $10.1 billion over the past five years.
Answer any industry question in minutes with our entire database at your fingertips.
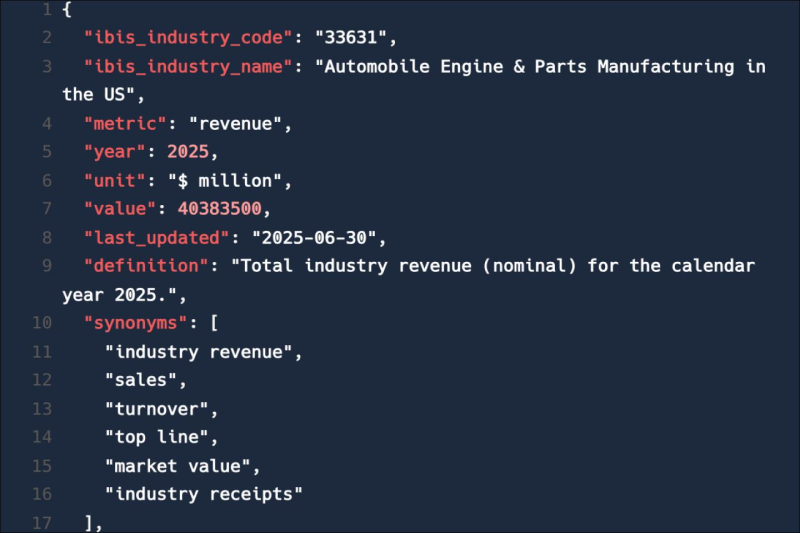
Feed trusted, human-driven industry intelligence straight into your platform.
Streamline your workflow with IBISWorld’s intelligence built into your toolkit.
IBISWorld's research coverage on the Clinical Trial Support Services industry in the United States includes market sizing, forecasting, data and analysis from 2015-2030. The most recent publication was released December 2025.
The Clinical Trial Support Services industry in the United States operates under the NAICS industry code OD4179. Companies specializing in clinical trial support services assist pharmaceutical companies, biotechnology firms, research institutions or contract research organizations (CROs) in the planning, execution, management and reporting of clinical trials. These services are designed to ensure that clinical trials are conducted efficiently, ethically and in compliance with regulatory requirements. Related terms covered in the Clinical Trial Support Services industry in the United States include contract research organization, clincial research associate, patent cliff, investigational new drug, regulatory submission, adverse event, phase i clinical trial, phase ii clinical trial, phase iii clinical trial and phase iv clinical trial.
Products and services covered in Clinical Trial Support Services industry in the United States include Support services for drug clinical trials, Support services for behavioral interventional studies and Support services for medical device interventional studies.
Companies covered in the Clinical Trial Support Services industry in the United States include Iqvia Holdings Inc., Parexel International Corp and Syneos Health, Inc.
The Performance chapter covers detailed analysis, datasets, detailed current performance, sources of volatility and an outlook with forecasts for the Clinical Trial Support Services industry in the United States.
Questions answered in this chapter include what's driving current industry performance, what influences industry volatility, how do successful businesses overcome volatility, what's driving the industry outlook. This analysis is supported with data and statistics on industry revenues, costs, profits, businesses and employees.
The Products and Markets chapter covers detailed products and service segmentation and analysis of major markets for the for the Clinical Trial Support Services industry in the United States.
Questions answered in this chapter include how are the industry's products and services performing, what are innovations in industry products and services, what products or services do successful businesses offer and what's influencing demand from the industry's markets. This includes data and statistics on industry revenues by product and service segmentation and major markets.
The Geographic Breakdown chapter covers detailed analysis and datasets on regional performance of the Clinical Trial Support Services industry in the United States.
Questions answered in this chapter include where are industry businesses located and how do businesses use location to their advantage. This includes data and statistics on industry revenues by location.
The Competitive Forces chapter covers the concentration, barriers to entry and supplier and buyer profiles in the Clinical Trial Support Services industry in the United States. This includes data and statistics on industry market share concentration, barriers to entry, substitute products and buyer & supplier power.
Questions answered in this chapter include what impacts the industry's market share concentration, how do successful businesses handle concentration, what challenges do potential industry entrants face, how can potential entrants overcome barriers to entry, what are substitutes for industry services, how do successful businesses compete with substitutes and what power do buyers and suppliers have over the industry and how do successful businesses manage buyer & supplier power.
The Companies chapter covers Key Takeaways, Market Share and Companies in the Clinical Trial Support Services industry in the United States. This includes data and analysis on companies operating in the industry that hold a market share greater than 5%.
Questions answered in this chapter include what companies have a meaningful market share and how each company is performing.
The External Environment chapter covers Key Takeaways, External Drivers, Regulation & Policy and Assistance in the Clinical Trial Support Services industry in the United States. This includes data and statistics on factors impacting industry revenue such as economic indicators, regulation, policy and assistance programs.
Questions answered in this chapter include what demographic and macroeconomic factors impact the industry, what regulations impact the industry, what assistance is available to this industry.
The Financial Benchmarks chapter covers Key Takeaways, Cost Structure, Financial Ratios, Valuation Multiples and Key Ratios in the Clinical Trial Support Services industry in the United States. This includes financial data and statistics on industry performance including key cost inputs, profitability, key financial ratios and enterprise value multiples.
Questions answered in this chapter include what trends impact industry costs and how financial ratios have changed overtime.
The Industry Data chapter includes 10 years of historical data with 5 years of forecast data covering statistics like revenue, industry value add, establishments, enterprises, employment and wages in the Clinical Trial Support Services industry in the United States.
More than 6,000 businesses use IBISWorld to shape local and global economies
We were able to supplement our reports with IBISWorld’s information from both a qualitative and quantitative standpoint. All of our reporting now features some level of IBISWorld integration.

IBISWorld delivers the crisp business knowledge we need to drive our business. Whether it be serving up our major clients, winning new business or educating on industry issues, IBISWorld brings real value.

IBISWorld has revolutionised business information — which has proved commercially invaluable to exporters, investors and public policy professionals in Australia and overseas.

When you’re able to speak to clients and be knowledgeable about what they do and the state that they operate in, they’re going to trust you a lot more.

The market size of the Clinical Trial Support Services industry in the United States is $10.1bn in 2026.
There are 2,098 businesses in the Clinical Trial Support Services industry in the United States, which has grown at a CAGR of 6.2 % between 2020 and 2025.
The Clinical Trial Support Services industry in the United States is unlikely to be materially impacted by import tariffs with imports accounting for a low share of industry revenue.
The Clinical Trial Support Services industry in the United States is unlikely to be materially impacted by export tariffs with exports accounting for a low share of industry revenue.
The market size of the Clinical Trial Support Services industry in the United States has been declining at a CAGR of 1.9 % between 2020 and 2025.
Over the next five years, the Clinical Trial Support Services industry in the United States is expected to grow.
The biggest companies operating in the Clinical Trial Support Services industry in the United States are Iqvia Holdings Inc., Parexel International Corp and Syneos Health, Inc.
Preparation of medical clinical trial services and Tracking and recording results and data are part of the Clinical Trial Support Services industry in the United States.
The company holding the most market share in the Clinical Trial Support Services industry in the United States is Iqvia Holdings Inc..
The level of competition is moderate and steady in the Clinical Trial Support Services industry in the United States.




